
The different types
Like all cancers, “endometrial cancer” actually corresponds to several very different diseases.
Several ways to classify them exist and combine.
The interest of classifying the different types of endometrial cancer makes it possible to specify their prognosis and to offer them an appropriate treatment.
The anatomopathological classification distinguishes the forms according to the appearance of the tissue and the cells.
We distinguish:
- Endometrioid cancers
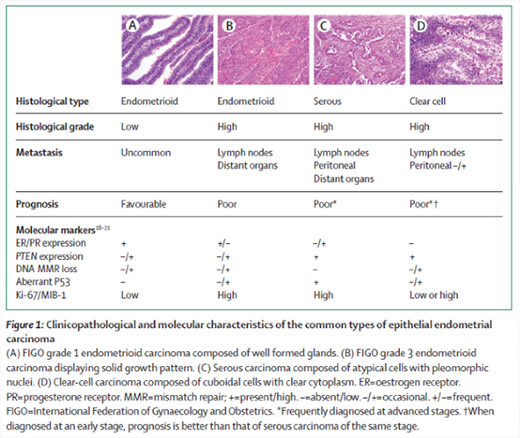
They are the most common (about 80% of endometrial cancers). They come from pre-cancerous lesions, induced by estrogen or an unfavorable estrogen/progesterone ratio: hyperplasia with atypia. Under the effect of estrogens, the normal endometrium can progress to simple hyperplasia, then hyperplasia with atypia, then cancer.
They are assigned a grade according to the microscopic appearance of the tumour, representative of their aggressiveness.
- Serous cancers
They look like ovarian cancers. They are aggressive and regularly associated with damage to the pelvic and abdominal peritoneum and lymph nodes.
They fall within the scope of rare cancers treated by the national network of Rare Gynecological Malignant Tumors (www.ovaire-rare. org).
- Clear cell cancers
They are rarer and fall within the scope of rare cancers treated by the national network of Rare Gynecological Malignant Tumors (www. ovaire-rare.org).
- Carcinosarcomas
They are also called Mixed Mullerian Tumors. It is a rare, aggressive disease (they fall within the scope of rare cancers covered by the national network of Rare Gynecological Malignant Tumors (www.ovaire-rare.org)).
They combine a carcinomatous component with a sarcomatous component.
- Mucinous cancers
- Squamous cell cancers
- Mixed cancers
- Undifferentiated cancers
Histogenic classification
It takes into account histopathological classification and molecular biology.
We distinguish
- - Type I cancers
We find endometrioid cancers and mucinous cancers.
These are the most common cancers (about 80% of endometrial cancers). They concern women in pre or peri-menopause. They are induced by estrogen. They come from the transformation of a normal endometrium into hyperplasia without atypia, then into hyperplasia with atypia and then into endometrioid carcinoma. Their grade is generally low and they hardly infiltrate the myometrium. Their prognosis is usually good.
- Type II cancers
It includes serous cancers, clear cell cancers and carcinosarcomas.
They are rarer (20% of cases) do not follow a pre-cancerous lesion such as hyperplasia with atypia, but an intraepithelial carcinoma. They concern postmenopausal women. They are high grade, deeply infiltrate the myometrium, are often associated with lymphovascular emboli and lymph node involvement. Their prognosis is worse.
The biological profile of type I and type II cancers is different (this table is taken from the article by Murali R & al, 2014):
:
 Molecular classification.
Molecular classification.
It comes from the study carried out as part of the Genome Atlas.
There are four different types: PolE (ultramutated), Unstable Microsatellites (hypermutated), low copy number, high copy number (The Cancer Genome Atlas Research Network, Nature 2013).
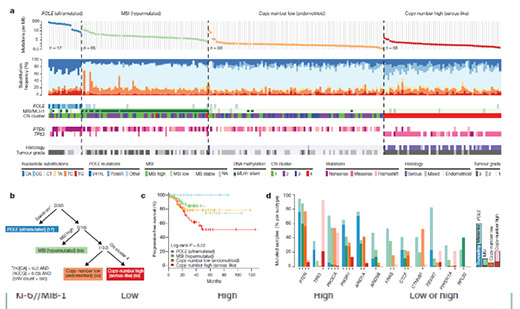 The first group is rare, but has a very good prognosis.
The first group is rare, but has a very good prognosis.
The second group corresponds to so-called “unstable” tumors (instability of microsatellite loci). This biological peculiarity corresponds to an anomaly in the length of short and repeated DNA sequences in the genome (microsatellites). It is due to the mutation or inactivation of a gene involved in DNA repair (repair of base mismatches during mitosis): hMLH1, hMSH2, hMSH6, PMS2. One of these genes can be mutated, we then speak of Lynch syndrome; or inactivated (age-related hMLH1 promoter methylation). These tumors respond to immunotherapies.
The fourth group very frequently has a mutation of the P53 gene, which brings these tumors closer to ovarian cancers, biologically.
Finally, there is a purely prognostic classification.
It was proposed by ESMO (European Society of Medical Oncology) in 2008 and has been maintained ever since. This is a pre-therapeutic classification that uses anatomopathological data (from biopsies or curettage) and radiological data (mainly from MRI).
Low-risk cancers,
These are grade 1 or 2 endometrioid cancers, infiltrating less than half the thickness of the myometrium and limited to the uterus.
These are the most common cancers. Their prognosis is good in the majority of cases.
Intermediate-risk cancers,
These are grade 3 endometrioid cancers, infiltrating less than half the thickness of the myometrium; or grade 1 or 2 endometrioid cancers infiltrating more than 50% of the thickness of the myometrium and limited to the uterus.
High-risk cancers,
It includes grade 3 endometrioid cancers infiltrating more than 50% of the thickness of the myometrium, non-endometrioid cancers (regardless of infiltration) limited to the uterus and cancers extending beyond the uterus.
How does endometrial cancer arise?
The main risk factors for endometrial cancer are:
- Age.
Endometrial cancer is rare before menopause. Type II cancers occur in older women than type I cancers.
- Hyperestrogenism.
This mainly concerns type I cancers
This excess estrogen can have several possible causes:
- Obesity
It is the most common cause of endogenous hyperestrogenism today. Androgens are converted to estrogen in adipose (fat) tissue. The amount of estrogen produced is proportional to the abundance of adipose tissue.
The geographic distribution of endometrial cancer overlaps with that of obesity.
- Diabetes
It is often associated with obesity, but is a risk factor in its own right.
- High blood pressure
It is also associated with obesity.
- Exogenous intakes of estrogens
Hormone treatment for menopause that does not include progestins was an important cause in the 1960s. However, this type of regimen has not been used for more than 40 years.
Tamoxifen, prescribed in the treatment of breast cancer, has also been implicated. However, the risk is low in absolute terms. But any bleeding that occurs in a woman treated with tamoxifen or who has been treated should be investigated.
- Polycystic ovary syndrome
- Alcoholism is also a contributing factor, through induced hyper-estrogenism.
- Heredity.
The main hereditary predisposition to endometrial cancer is Lynch syndrome (see specific tab).
Cowden syndrome, due to the mutation of the gene coding for the PTEN protein, is a rarer cause of hereditary endometrial cancer.
We also mention the history of pelvic radiotherapy.
The risk factors marked with an * are those on which it is possible to act to limit the individual or collective risk of developing endometrial cancer.
We can also act on these factors after treatment to limit the risk of recurrence.
Symptoms of endometrial cancer
There is no effective screening for endometrial cancer. This disease is therefore diagnosed when symptoms appear.
The main symptom of endometrial cancer is bleeding from the uterus.
- This bleeding occurs most often after menopause
- But the persistence of abnormal bleeding (too heavy or too frequent periods) before the menopause should also be investigated for this cancer.
Bleeding may be minimal or even almost absent, especially in women whose cervix is not very permeable (age, history of conization).
More rarely, a discharge of pus or clear liquid is observed.
Advanced stages may be associated with pelvic or abdominal pain.
The diagnosis
The first consultation is an important time.
It helps to guide the diagnosis by confirming the enduterine origin of the bleeding, prepares or takes the sample that will confirm the diagnosis and analyzes the general condition of the patient.
It is important to take stock of the general condition, with
- Age
o and search for frailty + / - onco-geriatrics consultation
o or conversely discussion of fertility preservation in the rare cases occurring in young women
- The search for obesity, the evaluation of its severity, the verification (or organization) of its management
- The search for high blood pressure and diabetes, the evaluation of its severity, the verification (or organization) of its management
- The search for other associated pathologies
- Identification of previous abdominal or pelvic procedures
- Search for personal and family history that may suggest Lynch syndrome (see specific tab)
The clinical examination is important since it verifies that the bleeding is of uterine origin, eliminating bleeding from the cervix, vagina or vulva.
It is possible to take an endometrial sample (endometrial biopsy) using a small cannula, in consultation.
The gynecological examination verifies that the tumor is “clinically” limited to the uterine body (the most common situation).
The abdominal examination verifies the absence of extension of the cancer.
It is also necessary to verify (or request) the realization of a mammogram and a cervical smear of screening if the age of the patient places her in the target population of the screening.
We also ask to stop any hormonal treatment.
It is important that this first consultation be carried out by a practitioner familiar with the pathology in order to gather all the important information from the outset and prescribe the right assessment from the outset.
Unless there is an ectopic anomaly noted by clinical examination, the only additional examination to be performed is a pelvic ultrasound.
This is a triage examination to distinguish women with a low risk of having a pathology from those who need to be explored further.
She is asked to measure the thickness of the endometrium.
The risk of secondarily diagnosing an endometrial or uterine cavity pathology is correlated to this thickness.
In premenopausal women, this thickness must be less than 8mm in the first part of the cycle and 10mm in the second part.
In premenopausal women, without hormonal treatment, the thickness should not exceed 6mm.
If the thickness is below the threshold, the risk of pathology is low. The bleeding is probably due to atrophy of the endometrium (mucosa that is too thin).
If the thickness is above the threshold, there is probably a pathology of the endometrium or of the uterine cavity and a second-line examination should be considered: diagnostic hysteroscopy and endometrial biopsy. It is useful to remember that in postmenopausal women, without hormonal treatment, the risk of diagnosing endometrial cancer following bleeding is less than 10%.
The other examinations are logically only requested in a second step, even if often the high probability of the diagnosis of endometrial cancer makes their prescription anticipated.
- Diagnostic hysteroscopy.
This examination consists of the introduction of a small diameter optical device (generally 3mm) into the uterine cavity, following the canal of the cervix.
Diagnostic hysteroscopy can be performed in consultation most often (with a medicinal preparation of the cervix to facilitate permeability). Otherwise, it is performed under anesthesia during outpatient hospitalization, especially when the cervix is not very permeable.
We can thus observe the contents of the cavity and identify a polyp, a fibroid, suspicious vegetation.
Finally, hysteroscopy guides the performance of the endometrial biopsy.
- A tissue sample (biopsy)
Most often it is an endometrial biopsy performed during the initial consultation or immediately following the hysteroscopy.
More rarely, a curettage is performed.
This levy
- gave the diagnosis: endometrial adenocarcinoma;
- and provides all the important information to decide on the necessary assessment and the start of treatment: histological type, grade if applicable.
The quality of this diagnosis is important because it determines the entire start of treatment.
The balance sheet
In the majority of cases, the disease presents as an endometrioid adenocarcinoma, clinically limited to the uterus.
Only one examination is necessary, a pelvic and abdominal MRI.
It provides a lot of information:
- confirmation that the tumor is limited to the uterine body
- myometrial invasion depth
- appearance of important lymph node territories in endometrial cancer (pelvis, aortico-caval)
Depending on the grade obtained by the endometrial biopsy and the depth of invasion of the myometrium given by the MRI, treatment will begin with
- a non-conservative total hysterectomy with sentinel lymph node sampling
- or non-conservative total hysterectomy with pelvic lymph node dissection and aortico-cellar
- these procedures are most often performed by laparoscopy or robot-assisted laparoscopy. More rarely, a laparotomy (classic opening of the abdomen) may be used, due to certain parameters (size of the uterus, associated medical pathologies, abdominal adhesions, etc.).
In the rarer cases where a serous cancer is diagnosed, or in the event of clinical abdominal extension or on the MRI for example, a thoraco-abdomino-pelvic scanner and a CA 125 marker assay will also be used.
We are thus looking for (or taking stock of) an associated abdominal extension.
The treatment will then be different, starting with an intervention (non-conservative total hysterectomy, omentectomy (removal of the omentum, fatty apron hanging from the stomach and the transverse colon), pelvic and aortico-caval dissections, appendectomy, etc. ). These operations are of the same type as those performed for ovarian cancer (see specific tab).
Treatment may also begin with chemotherapy, possibly followed by surgery.
We understand the importance of the initial assessment. It is necessary to know from the beginning the precise histological characteristics of the tumor and its clinical and imaging extension.
It is also important to know the general state and the associated pathologies (co-morbidity) because they can modify the management by preferring chemotherapy to a major intervention with a significant perioperative risk.
Hysterectomy. This is the removal of the uterus. In the case of endometrial cancer, it is called total (because it removes the cervix) and non-conservative (because it removes the ovaries and fallopian tubes).
It allows the removal of the tumor (which is inside the uterus) with a margin of safety compared to the remaining tissues.
She must respect the rules of oncological surgery: no uterine manipulator during the procedure, no morcellation of the uterus, etc.
Laparoscopy. The procedure is performed with small instruments (5mm in general) passed through trocars above the pubis. The surgeon sees inside the belly thanks to an optic coupled to a camera.
These procedures must be performed under general anesthesia and require the belly to be “inflated” with CO2 to move the intestine away from the uterus.
Procedures performed by laparoscopy last longer than procedures performed by laparotomy, but are associated with fewer complications during and after the procedure. The duration of hospitalization and convalescence are also shorter after laparoscopy. Finally, survival is comparable after laparoscopy or laparotomy. These data were validated by randomized clinical trials in the 1990s and early 2000s.
Robot-assisted laparoscopy. This is actually an improved laparoscopy. The operator is seated at a console which provides him with a High Definition 3-dimensional view. The instruments are not held directly by the surgeon's hand, but manipulated using ultra-precise controls.
The robot improves the precision and quality of complex procedures, especially in obese patients.
Sentinel lymph node. Lymph nodes are part of the surgical and anatomopathological assessment of most cancers. This makes it possible to estimate the prognosis and decide on treatments. Until recently, this lymph node removal was done by a dissection that removed a large number of lymph nodes.
The sentinel lymph node technique involves removing only the first lymph node(s) that drain a tumor.
This technique has the advantage of being less aggressive surgically, with in particular fewer complications (bleeding during the procedure, nerve or urinary wounds, large leg: lymphedema; pockets of lymph: lymphocele).
It also makes it possible to find lymph nodes outside the classic dissection areas.
Finally, this small number of lymph nodes can be analyzed much more precisely (ultrastaging) leading to the diagnosis of lymph node micrometastases or isolated tumor cells in the lymph nodes.
We therefore have additional and better quality information than with dissections, with fewer complications.
Technically, at the start of the procedure, a fluorescent product is injected into the cervix and the first fluorescent lymph nodes are detected (and removed). (products used in the early 2000s such as Patent Blue or isotopes are rarely used today)
The principle seems simple, but to be effective and reliable (remove the right lymph nodes), this technique requires minimal learning.
This technique is validated by clinical studies with good methodology for breast cancer and vulvar cancer.
It is still considered as an option in endometrial cancers and cervical cancers (an international validation clinical trial is in progress: SENTICOL III).
The treatments
For diseases limited to the uterine body, clinically and radiologically, treatment most often begins with surgery (hysterectomy and lymph node removal).
The anatomopathological examination then makes it possible to classify the disease according to 4 risk groups:
- low risk
- intermediate risk – low
- intermediate risk – high
- high risk
Depending on the risk group, the patient, a personalized treatment will be proposed at the end of a meeting: the multidisciplinary consultation meeting (RCP).
We can thus offer simple monitoring, vaginal brachytherapy, radiotherapy with brachytherapy, radio-chemotherapy + chemotherapy, chemotherapy, etc.
For diseases extending to the abdomen, the treatments will use chemotherapy, surgery, radiotherapy, according to a sequence adapted to the histological type of the disease, its extension and the patient.
The treatment recommendations generally follow those proposed by the ESMO (European Society of Medical Oncology), the French recommendations (SFOG – INCa, French Society of Gynecological Oncology) as well as the local recommendations (referential of the Institut Curie).< br>
Screening for Lynch syndrome, by immunohistochemical study of MMR (mismatch repair) proteins, is done in most cases. This makes it possible to identify patients at risk of presenting this syndrome; and to identify so-called “unstable” tumors that may benefit from immunotherapy in certain circumstances.
In any case, the management of obesity, diabetes, arterial hypertension, other pathologies, are also part of the care plan.
Multidisciplinary Consultation Meeting (RCP): each case being specific, the file of each patient is discussed during a meeting where pathologists, surgeons, onco-geriatricians, medical oncologists, radiotherapists, radiologists, etc. are present. in order to establish an appropriate therapeutic plan.
Monitoring
Monitoring is based on clinical examination and will be done alternately by doctors involved in the management of the disease (surgeon, oncologist, radiotherapist).
Patients classified in the low-risk group benefit from simple clinical examinations.
- Apart from the presence of symptoms, there is no indication to perform additional examinations (imaging, markers)
- the management of obesity, diabetes, high blood pressure, other pathologies, should not be forgotten
- the evaluation of urinary and digestive functions, as well as sexuality must also be addressed and, if necessary, benefit from appropriate care.
Monitoring becomes more complex as one belongs to a higher risk group. We can then combine imaging examinations or markers, depending on the initial disease and the treatments performed.
Lynch Syndrome
Lynch syndrome is a genetic disorder, also called HNPCC (Hereditary Non-Polyposis Colorectal Cancer) syndrome.
It is due to the mutation of one of the four genes hMLH1, hMSH2, hMSH6 or PMS2, which normally ensure the diagnosis and repair of DNA mismatches during its replication.
This mutation is transmitted in the family according to an autosomal dominant mode.
- Autosomal: transmission is not sex-linked. Both women and men can be carriers and transmitters.
- The risk of transmission is 50%
- Dominant: inheriting the mutation makes you exposed.
This predisposition leads to an increased risk of developing cancers, first and foremost nonpolyposis colorectal cancer (Human nonpolyposis colorectal cancer [HNPCC]), endometrial cancer in women, and to a lesser extent, cancer ovary, small intestine, stomach, urinary and hepatobiliary excretory tract. Thus, the cumulative risk of developing colorectal or endometrial cancer at age 80 is 20 and 40%, respectively.
Lynch syndrome should be suspected clinically when there is:
- At least three relatives with narrow-spectrum cancer
- One is first-degree related to one of the other two
- At least two generations affected
- At least one cancer diagnosed before the age of 50
- A familial adenomatous polyposis is excluded
- Tumors need to be checked
These criteria are defined as the Amsterdam Criteria 2.
This syndrome must also be systematically sought in the event of endometrial cancer occurring before the age of 50 or whatever the age if a first-degree relative has suffered from colorectal cancer or cancer of the LYNCH spectrum.
Anatomopathology laboratories routinely search for this syndrome using an immunohistochemical test that evaluates the expression of the proteins coded by these genes.
In the event of a positive test, in the event of an evocative family tree, an onco-genetic consultation is necessary as well as confirmation of the anomaly by a search for mutations in molecular biology.
Early identification of carriers of the mutation is important because:
- It allows screening or prevention of other cancers
- It makes it possible to diagnose affected relatives and offer them screening or prevention of the main cancers.
People affected by this syndrome must therefore benefit from specific and regular monitoring and follow-up.
Current recommendations call for the following monitoring:
- Performing a colonoscopy every two years from the age of 20.
- Performing an oesogastro-duodenal fibroscopy when performing the first colonoscopy
- Annual gynecological screening, including clinical examination, ultrasound and endometrial sampling
It is also possible to perform a non-conservative total hysterectomy from the age of 40 (INCa recommendations 2009). In practice, these interventions are mainly performed after 45 years, because the risk of endometrial cancer before this age is low; and because the consequences of hysterectomy are less at this age. Different proposals can nevertheless be made, depending on the age of onset of cancer in the family or the woman's personal history. The dialogue with the onco-genetics team is important.
Ongoing trials
Participation in therapeutic trials (“research protocols”) allows patients to benefit from therapeutic innovations currently being validated.
These studies are subject to multiple validations guaranteeing their scientific interest and respect for ethics, before being offered to patients.
The main open drug trials are:
- ROCSAN: addition of immunotherapy and a PARP inhibitor for the treatment of recurrent carcinosarcoma,
- UTOLA: use of olaparib in endometrial cancer recurrences.




